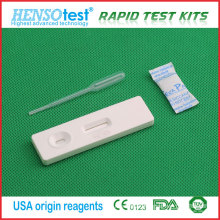
FSH Test Cassette Card Device
Basic Info
Model No.: HSL1104
Product Description
Operation Procedure One Step Casstte Style FSH Rapid Screen Test
PRODUCT NAME
Diagnosis kit for FSH(Follicle Stimulating Hormone) (Colloidal Gold)
PACKING SPECIFICATION
Casstte
DETECTION PRINCIPLE
The One Step FSH Test is a chromatographic immunoassay in-vitro diagnostic test for the qualitative detection. It adopts the principles of double antibody sandwich method to test the FSH in the urine.
SPECIMEN COLLECTION
1. Fresh urine does not require any special handing or pretreatment. Testing should be performed as soon as possible v after the specimen collection, preferably during the same day.
2. The specimen may be refrigerated at 2-8℃ for 3 days or frozen at -20℃ for a longer period of time. Specimens that have been refrigerated must be equilibrated to room temperature prior to testing. Specimens previously frozen must be thawed, equilibrated to room temperature, and mixed thoroughly prior to testing.
TEST PROCEDURE
1. When you are ready to begin testing, open the sealed pouch by tearing along the notch. Remove the test from the pouch.
2. Draw 2 or 3 drops sample into the pipette, and dispense it into the sample well on the cassette.
3. Wait 5-10 minutes and read result.
INTERPRETATION OF RESULTS
Positive: Two pink/purple bands form, and the color of the test band is equal to or more intense than that of the control/reference band.
Negative: One pink/purple band forms in the control region; no band is found in the test region, or the color of the test band is less intense than that of the control/reference band.
Invalid: If there is no pink/purple band in the control region, the test result is invalid. Retest the sample using a new device.
STORAGE AND STABILITY
The test kit can be stored at room temperature (2 to 30℃) in the sealed pouch for duration of shelf life. The test kits should be kept away from direct sunlight, moisture and heat.
PRECAUTION
1. For the vitro diagnostic use only.
2. Do not sue test kit beyond expiry date.
3. The test device should not be reused.
Detailed Images

 Code & Package
Code & Package
Production Facility  Company Introduction Henso Biotech Inc. > A member of Henso Medical Group company.
Company Introduction Henso Biotech Inc. > A member of Henso Medical Group company.
> A manufacturer and supplier of Rapid Test Kits: >> Fertility & Eugenic Test Pregnancy Test, Ovulation test, Menapause test, Toxoplasma test, CMV test... >> Infectious disease test Hepatitis test, Syphlisi (TP), Malaria test, Chlamydia test, Typhoid test, H.pylori test, Dengue test... >> Tumor markers test PSA test , FOB test, CEA test, AFP test >> DOA drug of abuse test KET, MET, MOR, AMP, COC, THC, BAR, MDMA, BUP, BZO, MTD, PCP, OPI, TCA... >> Urinalysis reagent test strip Leukocytes, Nitrite, Urobilinogen, Protein, pH, Blood, Specific Gravity, Ketone, Bilirubin, Glucose... >> Alcohol test, PH test
Product Category Certifications
Certifications  Contact us if you need more details on Fsh Test Cassette. We are ready to answer your questions on packaging, logistics, certification or any other aspects about Fsh Test Card、Fsh Test Device. If these products fail to match your need, please contact us and we would like to provide relevant information.
Contact us if you need more details on Fsh Test Cassette. We are ready to answer your questions on packaging, logistics, certification or any other aspects about Fsh Test Card、Fsh Test Device. If these products fail to match your need, please contact us and we would like to provide relevant information.
PRODUCT NAME
Diagnosis kit for FSH(Follicle Stimulating Hormone) (Colloidal Gold)
PACKING SPECIFICATION
Casstte
DETECTION PRINCIPLE
The One Step FSH Test is a chromatographic immunoassay in-vitro diagnostic test for the qualitative detection. It adopts the principles of double antibody sandwich method to test the FSH in the urine.
SPECIMEN COLLECTION
1. Fresh urine does not require any special handing or pretreatment. Testing should be performed as soon as possible v after the specimen collection, preferably during the same day.
2. The specimen may be refrigerated at 2-8℃ for 3 days or frozen at -20℃ for a longer period of time. Specimens that have been refrigerated must be equilibrated to room temperature prior to testing. Specimens previously frozen must be thawed, equilibrated to room temperature, and mixed thoroughly prior to testing.
TEST PROCEDURE
1. When you are ready to begin testing, open the sealed pouch by tearing along the notch. Remove the test from the pouch.
2. Draw 2 or 3 drops sample into the pipette, and dispense it into the sample well on the cassette.
3. Wait 5-10 minutes and read result.
INTERPRETATION OF RESULTS
Positive: Two pink/purple bands form, and the color of the test band is equal to or more intense than that of the control/reference band.
Negative: One pink/purple band forms in the control region; no band is found in the test region, or the color of the test band is less intense than that of the control/reference band.
Invalid: If there is no pink/purple band in the control region, the test result is invalid. Retest the sample using a new device.
STORAGE AND STABILITY
The test kit can be stored at room temperature (2 to 30℃) in the sealed pouch for duration of shelf life. The test kits should be kept away from direct sunlight, moisture and heat.
PRECAUTION
1. For the vitro diagnostic use only.
2. Do not sue test kit beyond expiry date.
3. The test device should not be reused.
Detailed Images


 Code & Package
Code & Package  Company Introduction Henso Biotech Inc. > A member of Henso Medical Group company.
Company Introduction Henso Biotech Inc. > A member of Henso Medical Group company.> A manufacturer and supplier of Rapid Test Kits: >> Fertility & Eugenic Test Pregnancy Test, Ovulation test, Menapause test, Toxoplasma test, CMV test... >> Infectious disease test Hepatitis test, Syphlisi (TP), Malaria test, Chlamydia test, Typhoid test, H.pylori test, Dengue test... >> Tumor markers test PSA test , FOB test, CEA test, AFP test >> DOA drug of abuse test KET, MET, MOR, AMP, COC, THC, BAR, MDMA, BUP, BZO, MTD, PCP, OPI, TCA... >> Urinalysis reagent test strip Leukocytes, Nitrite, Urobilinogen, Protein, pH, Blood, Specific Gravity, Ketone, Bilirubin, Glucose... >> Alcohol test, PH test
Product Category
 Certifications
Certifications  Contact us if you need more details on Fsh Test Cassette. We are ready to answer your questions on packaging, logistics, certification or any other aspects about Fsh Test Card、Fsh Test Device. If these products fail to match your need, please contact us and we would like to provide relevant information.
Contact us if you need more details on Fsh Test Cassette. We are ready to answer your questions on packaging, logistics, certification or any other aspects about Fsh Test Card、Fsh Test Device. If these products fail to match your need, please contact us and we would like to provide relevant information. Product Categories : Fertility & Eugenic Test
Other Products
Hot Products
200ml + 2000ml Disposable Urine MeterUrine Drainage Bag without outlet valveOne way (1 way) All Silicone Foley CatheterOne way (1 way) Latex Foley CatheterHenso Infusion SetHenso IV CatheterHenso Hypodermic NeedleHenso Disposable Syringe with Safety Cap (Needle with protective cap)HENSO Micro Blood Collection Tubes screw capHENSO Non-Vacuum Blood TubesHENSO Vacuum Blood Collection Plain TubeHENSO PVC Feeding TubeHENSO Soft-Tip Nasal CannulaHENSO T-shaped Silicone Perforated Wound DrainHENSO Colored Guedel AirwayHENSO Disposable PVC Medical Oxygen Mask